


What is the relationship between viscosity and the kinetic molecular theory of gases?
In Stock
$34.99
$29.99
Shipping and Returns Policy
- Deliver to United States » Shipping Policy «
- - Shipping Cost: $5.99
- - Handling time: 2-3 business days
- - Transit time: 7-10 business days
- Eligible for » Returns & Refund Policy « within 30 days from the date of delivery
Find similar items here:
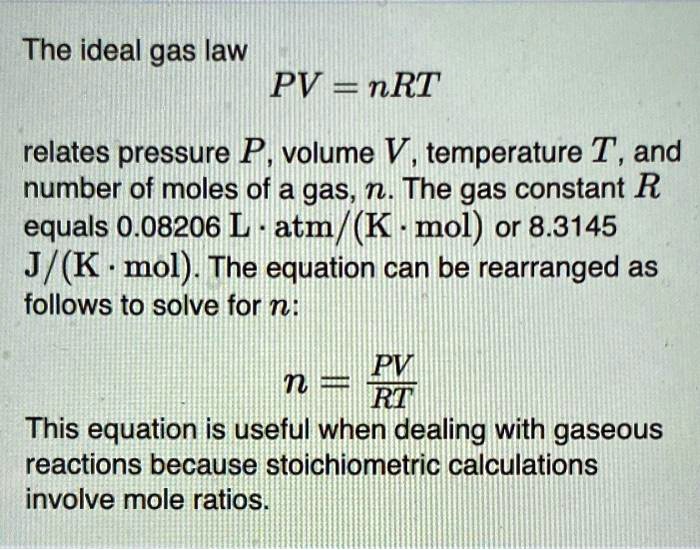
n pv rt what is r
- What are the effects of acceleration on the properties of gases?
- What are the sources and effects of common air pollutants?
- How does the ideal gas law relate to the study of phase transitions in gases?
- What is the composition of the interstellar medium?
- How can the ideal gas law be used to model gas flow?
- What are some applications of supercritical fluids?
- How does the ideal gas law apply to the behavior of gases in planetary atmospheres?
- What were some key experiments and discoveries that led to the formulation of the ideal gas law?
- What are equations of state for real gases other than the Van der Waals equation?
- What are some environmental implications of gas solubility in water, such as ocean acidification?
-
Next Day Delivery by USPS
Find out more
Order by 9pm (excludes Public holidays)
$11.99
-
Express Delivery - 48 Hours
Find out more
Order by 9pm (excludes Public holidays)
$9.99
-
Standard Delivery $6.99 Find out more
Delivered within 3 - 7 days (excludes Public holidays).
-
Store Delivery $6.99 Find out more
Delivered to your chosen store within 3-7 days
Spend over $400 (excluding delivery charge) to get a $20 voucher to spend in-store -
International Delivery Find out more
International Delivery is available for this product. The cost and delivery time depend on the country.
You can now return your online order in a few easy steps. Select your preferred tracked returns service. We have print at home, paperless and collection options available.
You have 28 days to return your order from the date it’s delivered. Exclusions apply.
View our full Returns and Exchanges information.
Our extended Christmas returns policy runs from 28th October until 5th January 2025, all items purchased online during this time can be returned for a full refund.
No reviews yet. Only logged in customers who have purchased this product may leave a review.
