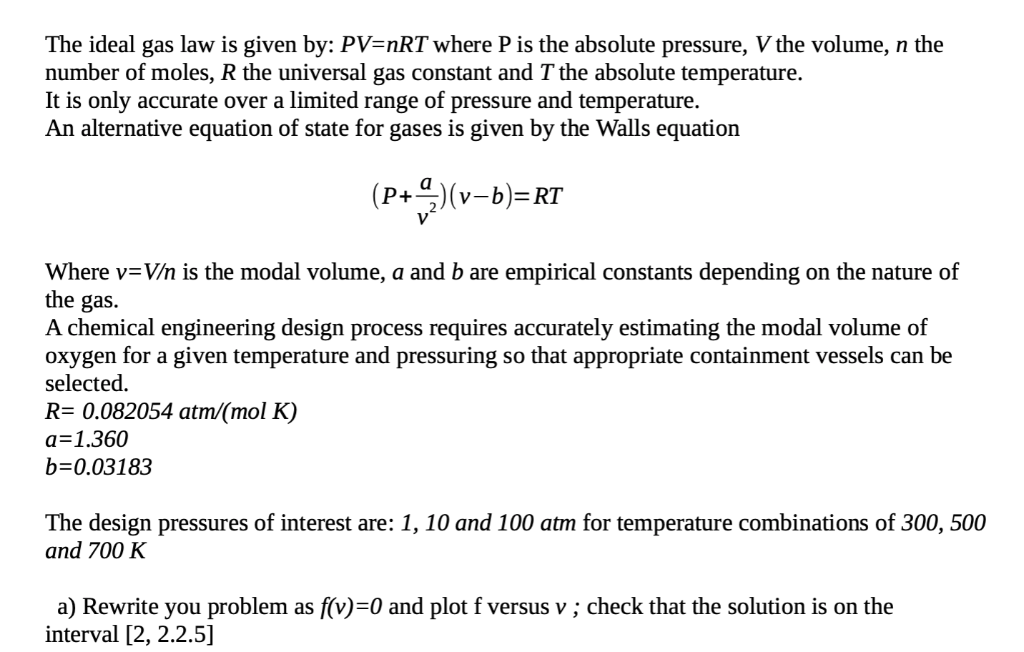

Can the ideal gas law be used to explain the behavior of atmospheric gases?
In Stock
$34.99
$29.99
Shipping and Returns Policy
- Deliver to United States » Shipping Policy «
- - Shipping Cost: $5.99
- - Handling time: 2-3 business days
- - Transit time: 7-10 business days
- Eligible for » Returns & Refund Policy « within 30 days from the date of delivery
Find similar items here:
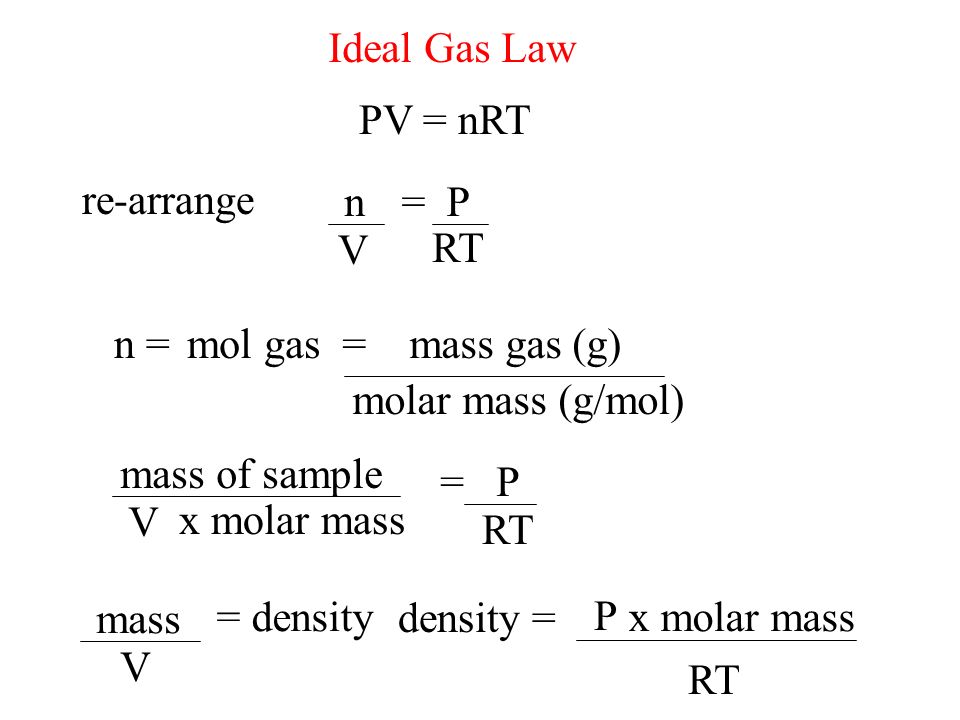
n pv rt what is r
- How does Graham's Law of Effusion provide evidence for the kinetic molecular theory of gases?
- How does the ideal gas law relate to the behavior of gases in high magnetic fields, such as in fusion reactors?
- What is the Maxwell-Boltzmann distribution of molecular speeds?
- What is the role of the refrigerant gas in the cooling cycle?
- What are the conditions required for nuclear fusion to occur?
- Is 'R' a universal constant?
- How do organisms living in these environments cope with the extreme pressures and temperatures?
- How does the ideal gas law relate to the behavior of gases in porous materials?
- How does the ideal gas law relate to the study of phase transitions in gases?
- What are some of the key assumptions of the ideal gas law that break down under different conditions?
-
Next Day Delivery by USPS
Find out more
Order by 9pm (excludes Public holidays)
$11.99
-
Express Delivery - 48 Hours
Find out more
Order by 9pm (excludes Public holidays)
$9.99
-
Standard Delivery $6.99 Find out more
Delivered within 3 - 7 days (excludes Public holidays).
-
Store Delivery $6.99 Find out more
Delivered to your chosen store within 3-7 days
Spend over $400 (excluding delivery charge) to get a $20 voucher to spend in-store -
International Delivery Find out more
International Delivery is available for this product. The cost and delivery time depend on the country.
You can now return your online order in a few easy steps. Select your preferred tracked returns service. We have print at home, paperless and collection options available.
You have 28 days to return your order from the date it’s delivered. Exclusions apply.
View our full Returns and Exchanges information.
Our extended Christmas returns policy runs from 28th October until 5th January 2025, all items purchased online during this time can be returned for a full refund.
No reviews yet. Only logged in customers who have purchased this product may leave a review.
