
How does the wave function of an electron in an atom depend on the quantum numbers n, l, and ml?
In Stock
$34.99
$29.99
Shipping and Returns Policy
- Deliver to United States » Shipping Policy «
- - Shipping Cost: $5.99
- - Handling time: 2-3 business days
- - Transit time: 7-10 business days
- Eligible for » Returns & Refund Policy « within 30 days from the date of delivery
Find similar items here:
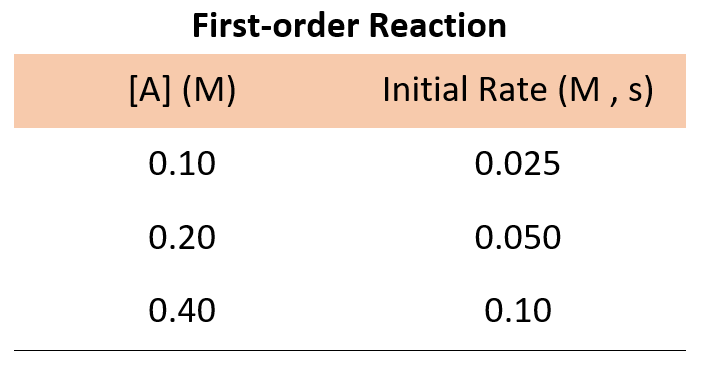
what is n in chemistry
- What are spin-orbit coupling and its effect on the energy levels and splitting of spectral lines, and how does it depend on n, l, and s?
- In electron microscopy, how does the interaction of electrons with a sample depend on their energy and the electronic structure (related to n) of the sample atoms?
- How does the work function of a metal relate to the energy of electrons at the Fermi level, which originates from atomic orbitals with various n values?
- In scanning tunneling microscopy (STM), the tunneling current between a sharp tip and a conducting surface depends on the electron density at the Fermi level of both the tip and the sample. How does the atomic structure and electronic states (related to n) of the surface influence the tunneling current and the resulting STM images?
- In catalysis, how does the interaction of reactants with a catalyst surface involve changes in their electronic states (related to n)?
- In spectroscopy techniques like NMR and EPR, does the principal quantum number play a direct role in the observed signals?
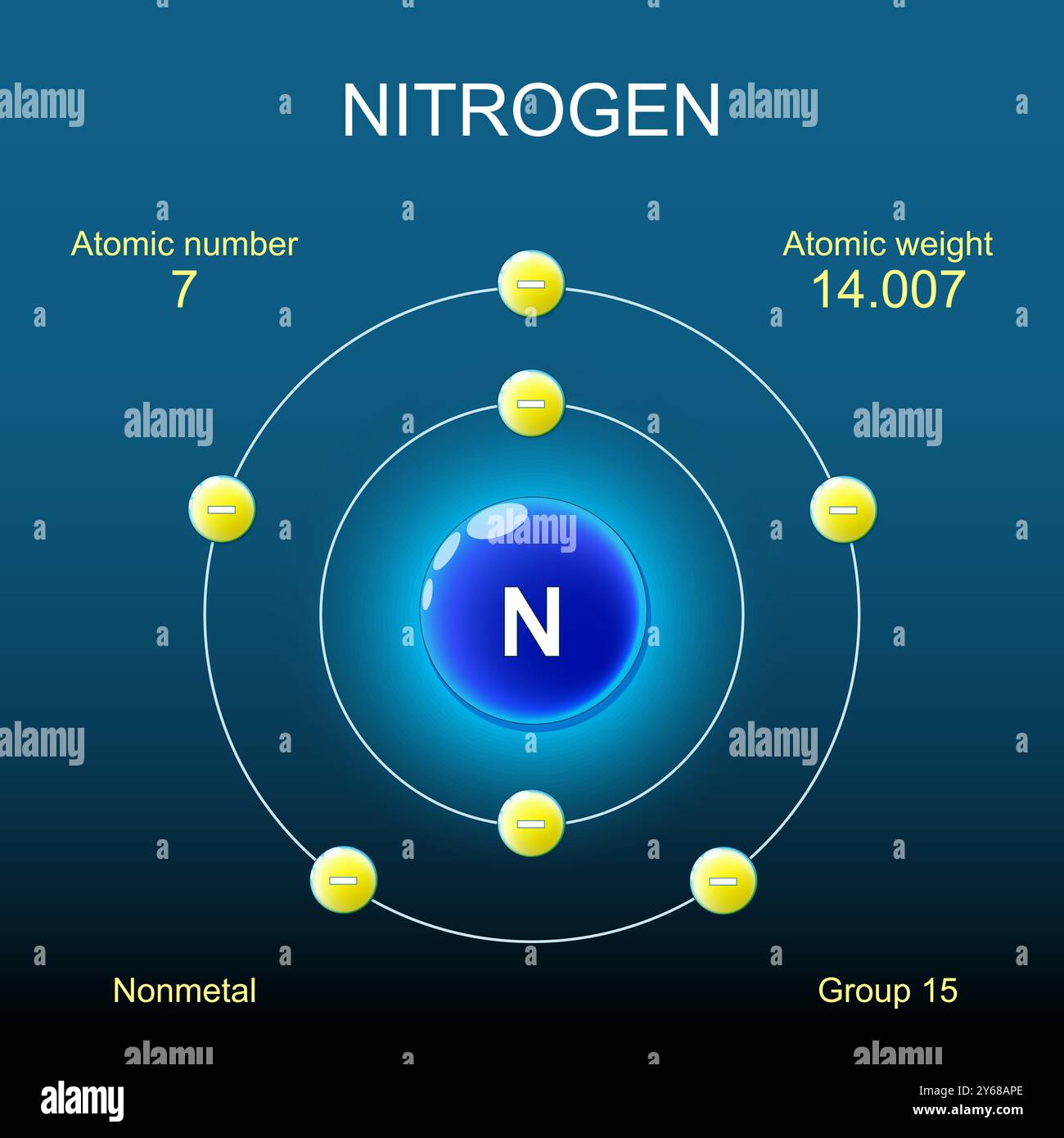
- How does the filling of these orbitals according to Hund's rule and the Pauli Exclusion Principle lead to the electron configurations of elements?
- How does the concept of resonance describe the delocalization of electrons in some molecules, and do these delocalized electrons occupy molecular orbitals formed from atomic orbitals with similar n values?
- How are lasers based on stimulated emission between specific energy levels with different n values?
- How does ionization energy generally vary across a period and down a group, and how is this related to n?
-
Next Day Delivery by USPS
Find out more
Order by 9pm (excludes Public holidays)
$11.99
-
Express Delivery - 48 Hours
Find out more
Order by 9pm (excludes Public holidays)
$9.99
-
Standard Delivery $6.99 Find out more
Delivered within 3 - 7 days (excludes Public holidays).
-
Store Delivery $6.99 Find out more
Delivered to your chosen store within 3-7 days
Spend over $400 (excluding delivery charge) to get a $20 voucher to spend in-store -
International Delivery Find out more
International Delivery is available for this product. The cost and delivery time depend on the country.
You can now return your online order in a few easy steps. Select your preferred tracked returns service. We have print at home, paperless and collection options available.
You have 28 days to return your order from the date it’s delivered. Exclusions apply.
View our full Returns and Exchanges information.
Our extended Christmas returns policy runs from 28th October until 5th January 2025, all items purchased online during this time can be returned for a full refund.
No reviews yet. Only logged in customers who have purchased this product may leave a review.
