


What do magic numbers indicate about nuclear stability?
In Stock
$34.99
$29.99
Shipping and Returns Policy
- Deliver to United States » Shipping Policy «
- - Shipping Cost: $5.99
- - Handling time: 2-3 business days
- - Transit time: 7-10 business days
- Eligible for » Returns & Refund Policy « within 30 days from the date of delivery
Find similar items here:
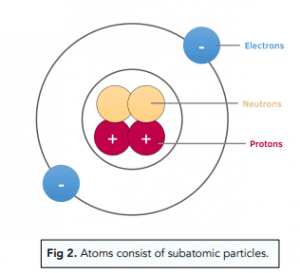
what are the subatomic particles of an atom
- What are electroweak symmetry breaking and the Brout-Englert-Higgs mechanism? How do the masses of the W and Z bosons arise from the Higgs mechanism?
- How do Feynman diagrams represent interactions between subatomic particles?
- How do isotopes of an element differ? What are isobars?
- What is magnetic moment in subatomic particles? Which subatomic particles have a magnetic moment?
- What is an antigluon? What is the role of neutrinos in the subatomic world?
- What are the constituent quarks of a neutron?
- What are reductionism and emergence in the context of understanding matter from its fundamental constituents? What is the role of quantum mechanics in shaping our understanding of reality at the smallest scales?
- Has proton decay ever been observed? What are the implications of a theory of everything for our understanding of subatomic particles?
- What are supernovae?
- What are baryons? Which subatomic particles are classified as baryons?
-
Next Day Delivery by USPS
Find out more
Order by 9pm (excludes Public holidays)
$11.99
-
Express Delivery - 48 Hours
Find out more
Order by 9pm (excludes Public holidays)
$9.99
-
Standard Delivery $6.99 Find out more
Delivered within 3 - 7 days (excludes Public holidays).
-
Store Delivery $6.99 Find out more
Delivered to your chosen store within 3-7 days
Spend over $400 (excluding delivery charge) to get a $20 voucher to spend in-store -
International Delivery Find out more
International Delivery is available for this product. The cost and delivery time depend on the country.
You can now return your online order in a few easy steps. Select your preferred tracked returns service. We have print at home, paperless and collection options available.
You have 28 days to return your order from the date it’s delivered. Exclusions apply.
View our full Returns and Exchanges information.
Our extended Christmas returns policy runs from 28th October until 5th January 2025, all items purchased online during this time can be returned for a full refund.
No reviews yet. Only logged in customers who have purchased this product may leave a review.
