

How do theoretical physicists develop new models and theories to describe subatomic phenomena? What is the process of formulating and testing new ideas in particle physics?
In Stock
$34.99
$29.99
Shipping and Returns Policy
- Deliver to United States » Shipping Policy «
- - Shipping Cost: $5.99
- - Handling time: 2-3 business days
- - Transit time: 7-10 business days
- Eligible for » Returns & Refund Policy « within 30 days from the date of delivery
Find similar items here:
what are the subatomic particles of an atom
- Why is the Higgs boson's mass so much smaller than the Planck mass, and how might theories beyond the standard model address this hierarchy problem?
- Why are precision measurements important for testing the standard model?
- What role do protons play in the chemical identity of an element?
- What are the quark constituents of an antiproton? What is the antiparticle of a neutron?
- How can experiments on Earth help us understand the subatomic processes occurring in distant astronomical objects? What is neutrino astronomy?
- What is astrophysics?
- How does the trigger system select interesting events? What is data acquisition in particle physics experiments?
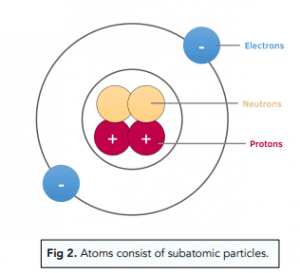
- What components make up the internal structure of an atom?
- What are magnetic monopoles?
- What are grid computing networks used for in particle physics? What is open science in the context of particle physics research?
-
Next Day Delivery by USPS
Find out more
Order by 9pm (excludes Public holidays)
$11.99
-
Express Delivery - 48 Hours
Find out more
Order by 9pm (excludes Public holidays)
$9.99
-
Standard Delivery $6.99 Find out more
Delivered within 3 - 7 days (excludes Public holidays).
-
Store Delivery $6.99 Find out more
Delivered to your chosen store within 3-7 days
Spend over $400 (excluding delivery charge) to get a $20 voucher to spend in-store -
International Delivery Find out more
International Delivery is available for this product. The cost and delivery time depend on the country.
You can now return your online order in a few easy steps. Select your preferred tracked returns service. We have print at home, paperless and collection options available.
You have 28 days to return your order from the date it’s delivered. Exclusions apply.
View our full Returns and Exchanges information.
Our extended Christmas returns policy runs from 28th October until 5th January 2025, all items purchased online during this time can be returned for a full refund.
No reviews yet. Only logged in customers who have purchased this product may leave a review.
